Home > Press > Nanoresearchers challenge dogma in protein transportation in cells
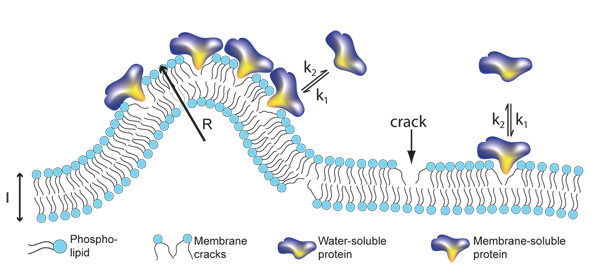 |
| Signalling proteins like to bind to cracks in membranes. The more curved the membrane is, the more places for binding of proteins. This model relates for the first time the size of transport container with the amount of bound protein. |
Abstract:
New data on signalling proteins, called G proteins, may prove important in fighting diseases such as cardiovascular, neurodegenerative disorders, and cancer. For many decades scientists have puzzled on "How signalling proteins transport and organize in specific areas of the cell?" Researchers from Nano-Science Center and Department of Neuroscience and Pharmacology provide yet unrecognized clues to solve this mystery.
Nanoresearchers challenge dogma in protein transportation in cells
Copenhagen | Posted on September 22nd, 2009We now begin to understand how signalling proteins recognize and transport to certain areas of the cell and get a more clear insight on the mechanism of major cellular processes such as cell signalling and growth. This valuable knowledge could be used in the future to understand and cure disease such as depression and Alzheimer's explains Associate Professor Dimitrios Stamou, Nano-Science Center and Department of Neuroscience and Pharmacology, who led the work.
Cells depend critically on their ability to selectively, transport and isolate proteins in specific areas. Earlier ideas that proposed proteins to move around in the cell by recognizing nanoscale patches in their surrounding membrane, also called lipid rafts, are currently under intense debate. However researchers from Nano-Science Center found a new unsuspected mechanism based on the shape of the membrane and just had their results published in the prominent scientific journal Nature Chemical Biology.
Attractive curves on the nanoscale
Like all other materials, cell membranes will crack when bend. Membranes however show a unique property: bending them more and more does not create bigger cracks but simply many more cracks of the same size. It turns out certain important proteins "like" to bind in these cracks therefore the curved parts of a membrane become a good place for them to "meet" each other and thus perform the complicated tasks that need many different proteins working side by side.
- We were very surprised that it is the number of cracks in the membrane that determines how many proteins are bound. Up until now researchers in the field thought that the crucial element was the proteins ability and "desire" to bind to the membrane, also called the affinity. Our data speaks against that, explains Nikos Hatzakis, Nano-Science Center and Department of Chemistry.
The model is general
In cells proteins are travelling around in small vesicles - a kind of soap bubbles that like cells are surrounded by membranes. The researchers made vesicles of different sizes in the laboratory and tested how different types of proteins bound to the vesicle membrane. They observed that the smaller the size of the vesicle, and more curved the membrane, the higher the number of cracks available and therefore the greater the number of proteins that can be bound pr. surface area.
- The moment we understood that the most critical parameter in our observations was membrane-shape we immediately thought that maybe we found a general mechanism that would apply to many other types of proteins apart from the ones we were studying. So we tested G proteins that are important signalling proteins attached to the membrane in a different way, using a lipid anchor. Our data confirmed that the model was indeed general, explains Vikram Bhatia, Nano-Science Center and Department of Nanoscience and Pharmacology.
- Unravelling the overarching importance of membrane-shape for the localization of literally hundreds of important signalling proteins will prove critical to our understanding of a plethora of biological process many of which are directly linked to important diseases, emphasises Associate Professor Dimitrios Stamou.
####
About University of Copenhagen
With over 37,000 students and more than 7,000 employees, the University of Copenhagen is the largest institution of research and education in Denmark.
For more information, please click here
Contacts:
Associate Professor Dimitrios Stamou, Nano-Science Center and the Department of Neuroscience and Pharmacology
Tel.: +45 41 16 04 68
Communication Officer
Gitte Frandsen
Nano-Science Center
Tel.: +45 28 75 04 58
Copyright © University of Copenhagen
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








