Home > Press > Luna Works with the National Institutes of Health Exploring New Diagnostics for Heart Attack Prevention
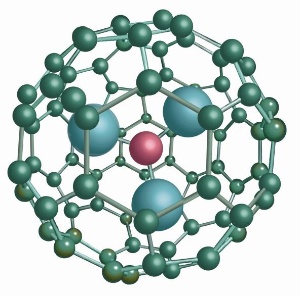 |
| Luna Innovations has been awarded a grant from the National Heart, Lung, and Blood Institute for the development of a new diagnostic agent for coronary artery disease. Luna is investigating a carbon nanomaterial-based contrast agent, using its exclusive TRIMETASPHERE(R) platform, which may provide the ability to use magnetic resonance imaging (MRI) to reveal plaque buildup in the arterial walls of the heart. Luna's contrast agent is anticipated to give a physician improved diagnostic performance and safety over gadolinium-containing contrast agents currently used in MRI. (Graphic: Business Wire) |
Abstract:
National Heart, Lung, and Blood Institute Awards Funding for Detection Tool Identifying Arterial Wall Plaque Buildup
Luna Works with the National Institutes of Health Exploring New Diagnostics for Heart Attack Prevention
ROANOKE, VA | Posted on November 29th, 2007Luna Innovations Incorporated (NASDAQ:LUNA) has been awarded a grant from the National Heart, Lung, and Blood Institute to develop a new diagnostic agent that could improve the diagnosis of coronary artery disease (CAD), a condition that causes most heart attacks. Luna is investigating a carbon nanomaterial-based contrast agent, using its exclusive TRIMETASPHERE® platform, which may provide the ability to use magnetic resonance imaging (MRI) to reveal plaque buildup in the arterial walls of the heart and possibly provide fine structural detail of the plaque.
Coronary arteries supply blood and oxygen to the heart. CAD occurs when the arteries harden and narrow as plaque builds up on the interior walls, a condition known as atherosclerosis. In most people, plaque is stable, however; plaque can break off and form a blood clot which may block the flow of blood to the heart ultimately, leading to a heart attack. CAD is the most common type of heart disease and is the leading cause of death in the United States. Technologies that can allow medical personnel to better assess how much plaque buildup has occurred can improve the management of patients with CAD.
Currently, CAD is most-commonly diagnosed through cardiac catheterization angiography. In this method, a flexible tube is passed through an artery in the groin or arm to reach the coronary arteries. An x-ray blocking dye is then injected through the tube into the coronary arteries that allows the doctor to see by x-ray obstructions that hinder or prevent flow of blood through the heart and the location of blockages.
"Luna's MRI contrast agent targeted to arterial plaque may provide information that cannot be obtained with x-rays, such as the status of plaque in smaller arteries," explained Robert Lenk, President of Luna's nanoWorks division in Danville, VA. "Recent evidence, such as the WISE trial, a study on Women's Ischemica Syndrome Evaluation sponsored by the National Institutes of Health, indicated that plaque buildup in smaller arteries is important in managing CAD, especially for women's health."
"Our company is engaged in the discovery, licensing and development of unique intellectual property, with an eye on moving science to solutions," said Kent Murphy, CEO and Chairman of Luna Innovations. "Our Trimetasphere is a metallofullerene technology that has a unique behavior enabling us to improve and expand product performance in many areas, of which the most important is MRI."
Additionally, Luna's contrast agent under development is anticipated to give a physician improved diagnostic performance and safety over current gadolinium-containing contrast agents used in MRI. Preliminary tests indicate that the unique structure of the TRIMETASPHERE®-based agent encloses the metallic signal molecule in such a way that it cannot escape and cause harm to the patient. This enhanced safety is due to the nature of the TRIMETASPHERE® molecule, how it behaves in the magnetic field, and the encapsulation of the metals protecting patients from metal poisoning.
The introduction of TRIMETASPHERE®-based contrast agents may help prevent the recently discovered disease, Nephrogenic Systemic Fibrosis / Nephrogenic Fibrosing Dermopathy (NSF/NFD), which has been attributed to current gadolinium-based contrast agents used in MRI procedures. MRI is a non-invasive diagnostic procedure that enables physicians to visualize internal organs through magnetic interactions. This procedure may be enhanced by the use of contrast agents that improve the resolution of soft tissue structures inside the patient. However, some MRI contrast agents can be harmful to a patient. Since June 2006, the U.S. Food and Drug Administration has been reviewing reports about patients who have developed NSF/NFD after receiving a gadolinium-based contrast agent during an MRI scan. The cause of this rare and dangerous condition is not fully understood. NSF/NFD can cause severe joint problems, skin problems, kidney failure, or renal failure. In May, Federal health officials issued a black box warning for the current gadolinium-based contrast agents used in MRI to heighten awareness about the risk of NSF/NFD in patients with kidney problems. Luna's new technology, using the carbon nanomaterial known as TRIMETASPHERE®, may eliminate this problem.
####
About Luna Innovations
Luna Innovations Incorporated develops and manufactures new-generation products for the healthcare, telecommunications, energy and defense markets. Our products are used to measure, monitor, protect and improve critical processes in the markets we serve. Through its disciplined commercialization business model, Luna has become a recognized leader in transitioning science to solutions. Luna is headquartered in Roanoke, Virginia.
Luna’s nanoWorks Division has a world-class nanomaterial manufacturing facility in Danville, Virginia and is developing products empowered by nanomaterials. Luna’s exclusive TRIMETASPHERE® carbon nanomaterial is a novel composition of matter providing unique physical, chemical, thermal, magnetic, biological, optical and electronic properties. With an initial application focused on medical imaging, product R&D also includes pharmaceuticals, solar cells and composites.
Forward Looking Statements:
This release includes information that constitutes “forward-looking statements” made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995, including statements regarding Luna’s TRIMETASPHERE® product as well as the expected capabilities, performance and potential market acceptance of such product. Statements that describe Luna’s business strategy, goals, objectives, prospects, opportunities, plans or intentions are also forward-looking statements. Actual events or results may differ materially from the expectations expressed in such forward-looking statements as a result of various factors, including risks and uncertainties, many of which are beyond the control of Luna Innovations. Factors that may affect the future results of Luna Innovations are set forth in its Annual Report on Form 10-K, its periodic reports on Forms 10-Q and other filings with the Securities and Exchange Commission (“SEC”). Such filings are available at the SEC’s website at http://www.sec.gov , and at the company’s website at http://www.lunainnovations.com . The statements made in this release are based on information available to the company as of the date of this release and Luna Innovations undertakes no obligation to update any of the forward-looking statements after the date of this release.
The project described was supported by Grant Number R43HL087578 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
For more information, please click here
Contacts:
Luna Innovations Incorporated
Media Contact:
Karin Clark, 1-540-769-8400
or
Investor Contact:
Qorvis Communications
Sally Beerbower, 1-703-744-7800
Copyright © Business Wire 2007
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Grants/Sponsored Research/Awards/Scholarships/Gifts/Contests/Honors/Records
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
![]() New discovery aims to improve the design of microelectronic devices September 13th, 2024
New discovery aims to improve the design of microelectronic devices September 13th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








