Home > Press > New Technology Illuminates Protein Interactions in Living Cells
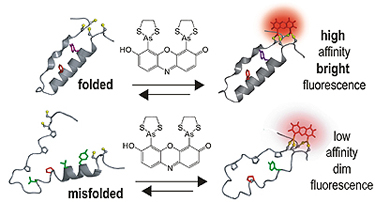 |
| When a target protein is folded correctly, “tags” come together so that the dye binds with high affinity and fluoresces brightly; misfolded proteins have low affinity for the dye. |
Abstract:
— While fluorescence has long been used to tag biological molecules, a new technology developed at Yale allows researchers to use tiny fluorescent probes to rapidly detect and identify protein interactions within living cells while avoiding the biological disruption of existing methods, according to a report in Nature Chemical Biology.
New Technology Illuminates Protein Interactions in Living Cells
New Haven, CT | Posted on November 11th, 2007Proteins are commonly tagged using variants of the "green fluorescent protein" (GFP), but these proteins are very large and are often toxic to live cells. They also tend to aggregate, making them difficult to work with and monitor. This new methodology uses the fluorescence emitted by a small molecule, rather than a large protein. It gives researchers a less disruptive way to capture images of the intricate contacts between folded regions of an individual protein or the partnerships between proteins in a live cell.
"Our approach bypasses many of the problems associated with fluorescent proteins, so that we can image protein interactions in living cells," said senior author Alanna Schepartz, the Milton Harris Professor of Chemistry, and Howard Hughes Medical Institute Professor at Yale. "Using these molecules we can differentiate alternative or misfolded proteins from those that are folded correctly and also detect protein partnerships in live cells."
Each protein is a three-dimensional structure created by "folding" its linear chain of amino acids. Usually only one shape "works" for each protein. The particular shape a protein takes depends on its amino acids and on other processes within the cell.
Schepartz and her team devised their new tagging system using small molecules, called "profluorescent" biarsenal dyes. These molecules easily enter cells and become fluorescent when they bind to a specific amino acid tag sequence within a protein. While these compounds have been used for about a decade to bind single proteins, this is the first time they have been used to identify interactions between proteins.
The researchers' strategy was to split the amino acid tag for the dye into two pieces, locating each piece of the tag far apart in the chain of a protein they genetically engineered and expressed in the cells. Then they monitored cells exposed to the dye. Where the protein folded correctly, the two parts of the tag came together and the fluorescent compound bound and lit up. There was no signal unless the protein folded normally.
"This method of detection can provide important insights into how proteins choose their partners within the cell — choices that may be very different from those made in a test tube," said Schepartz. She emphasizes that this technology does not monitor the process of protein folding — but, rather "sees" the protein conformations that exist at a given time.
"In theory, our technique could be used to target and selectively inactivate specific protein complexes in the cell, as therapy, or to visualize conformations at very high resolution for diagnostic purposes," said Schepartz. She speculates that the technology could be applied to detection strategies that identify protein misfolding in neurodegenerative diseases like Alzheimer's or Parkinson's.
Other authors on the paper are Nathan W. Luedtke, Rachel J. Dexter and Daniel B. Fried from the Schepartz lab at Yale. Funding from the Howard Hughes Medical Institute and the National Institutes of Health supported the research.
Citation: Nature Chemical Biology: (early online) 04 November 2007 | doi:10.1038/nchembio.2007.49
####
About Yale University
Yale University comprises three major academic components: Yale College (the undergraduate program), the Graduate School of Arts and Sciences, and the professional schools. In addition, Yale encompasses a wide array of centers and programs, libraries, museums, and administrative support offices. Approximately 11,250 students attend Yale.
For more information, please click here
Contacts:
Janet Rettig Emanuel
203-432-2157
Copyright © Yale University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








