Home > Press > Lab-on-a-Chip Device from Berkeley Lab to Speed Proteomics Research
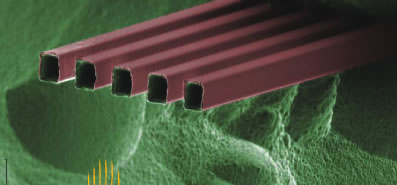 |
| This zoom-in Scanning Electron Microscope image shows a five-nozzle M3 emitter, where each nozzle measures 10x12 microns. |
Abstract:
In recent years, the science of biology has been dominated by genomics - the study of genes and their functions. The genomics era is now making way for the era of proteomics - the study of the proteins that genes encode.
Lab-on-a-Chip Device from Berkeley Lab to Speed Proteomics Research
Berkeley, CA | Posted on May 2nd, 2007Future proteomics research should see a substantial acceleration with the development of a new device that provides the first monolithic interface between mass spectrometry and silicon/silica-based microfluidic "lab-on-a-chip" technologies. This new device, called a multinozzle nanoelectrospray emitter array, was developed by scientists with the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab).
"Proteomics has become an indispensable tool in biological research, be it diagnostics, therapeutics, bioenergy or stem cell research, and mass spectrometry is proteomics' enabling technology," said Daojing Wang, a scientist with Berkeley Lab's Life Sciences Division who leads the proteomics research group and was the principal investigator behind the development of the multinozzle nanoelectrospray emitter.
"Lab-on-a-chip technology has enormous potential for proteomics research," Wang said, "but for this potential to be fully realized, a major advance in interfacing microfluidics with mass spectrometry is needed. Our device provides that interface."
Wang and Peidong Yang, a leading nanoscience authority with Berkeley Lab's Molecular Foundry and Materials Sciences Division, and also a chemistry professor with the University of California's Berkeley campus, co-authored a paper on this work which is being published by the American Chemical Society (ACS). The paper, which is now available in the on-line version. is entitled: "Microfabricated Monolithic Multinozzle Emitters for Nanoelectrospray Mass Spectrometry."
Other authors of the ACS paper were Woong Kim, a postdoctoral fellow in the Molecular Foundry, and Mingquan Guo, a postdoctoral fellow in the Life Sciences Division.
When the Human Genome Project was completed in 2003, giving scientists a complete catalogue of human DNA, the next big effort focused on genomics, identifying DNA sequences that code for proteins, aka, genes. With the identification of each and every new gene, the emphasis shifts to determining the biochemical function of its associated proteins.
All biological cells are constructed from aggregations of proteins that interact with other protein aggregations like an elaborate, finely choreographed network of interdependent machines. This biomolecular machinery also controls nearly every chemical process inside a cell, and forms much of the connectivity that enable cells to come together into tissues and organs. One of the first steps in proteomics research is to determine the identity and modifications of individual proteins that make up a cell or tissue sample. The principal means of doing this is through mass spectrometry.
Mass spectrometers use a combination of ionization and magnets to separate a protein's constituent peptides. Detection and analysis of this mass spectrum can then be used to identify the protein and quantify its presence in a sample. The most popular technique today for ionizing a protein's constituents for mass spectrometry is to liquefy the protein and send it through electrically charged capillaries - a technique known as electrospray ionization. One of the best candidates for high throughput integration of the detection and analysis processes is to interface the mass spectrometers with lab-on-a-chip technology, where biological fluids are introduced onto a microprocessor chip. However, microfluidic analysis of proteins has been a separate process from mass spectrometry - until now.
"Ours is the first report of a silicon/silica microfluidic channel that is integrated monolithically with a multinozzle nanoelectrospray emitter," said Wang. "This paves the way for the large scale integration of mass spectrometry and lab-on-a-chip analysis in proteomics research."
Each emitter consists of a parallel array of silica nozzles protruding out from a hollow silicon sliver with a conduit size of 100 x 10 microns. Multiple nozzles (100 nozzles per millimeter was a typical density) were used rather than single nozzles in order to reduce the pressure and clogging problems that arise as the microfluidic channels on a chip downsize to a nanometer scale. The emitters and their nozzles were produced from a silicon wafer, with the dimension and number of nozzles systematically and precisely controlled during the fabrication process. Fabrication required the use of only a single mask and involved photolithographic patterning and various etching processes.
Said Peidong Yang, "Once integrated with a mass spectrometer, our microfabricated monolithic multinozzle emitters achieved a sensitivity and stability in peptide and protein detection comparable to commercial silica-based capillary nanoelectrospray tips. This indicates that our emitters could serve as a critical component in a fully integrated silicon/silica-based micro total analysis system for proteomics."
Added Daojing Wang, "This is also the first report of a multinozzle emitter that can be fabricated through standard microfabrication processes. In addition to having lower back pressure and higher sensitivity, multinozzle emitters also provide a means to systematically study the electrospray ionization processes because the size of each nozzle and density of nozzles on the emitters can be adjusted."
According to Wang and Yang, the fabrication and application of the microfabricated monolithic multinozzle emitters, called "M3 emitters" for short, could be commercialized immediately and should be highly competitive with current silica capillary emitters in terms of cost and mass production.
"We are now in the process of creating a chip that integrates sample processing and preparation as well as detection and analysis," said Wang. "The ability to perform the full process on a single chip has enormous commercial potential."
Berkeley lab has filed for a patent on this technology. The research was supported by a grant from the National Institutes of Health, with some of the work done at Berkeley Lab's Molecular Foundry, which is supported by the Office of Science in the U.S. Department of Energy.
####
About Lawrence Berkeley National Laboratory
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California. Visit our Website at http://www.lbl.gov .
Additional information
* Daojing Wang can be reached by e-mail at
Peidong Yang can be reached by e-mail at
* A copy of the ACS paper describing this research can be viewed at
http://pubs.acs.org/cgi- bin/asap.cgi/ancham/asap/pdf/ac070010j.pdf
* For more about Daojing Wang visit the Website at
http://cancer.ucsf.edu/people/wang_daojing.php
* For more about Peidong Yang, visit the Website at
http://www.cchem.berkeley.edu/pdygrp/main.html
For more information, please click here
Contacts:
Lynn Yarris
(510) 486-5375
Daojing Wang
Peidong Yang
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Patents/IP/Tech Transfer/Licensing
![]() Getting drugs across the blood-brain barrier using nanoparticles March 3rd, 2023
Getting drugs across the blood-brain barrier using nanoparticles March 3rd, 2023
![]() Metasurfaces control polarized light at will: New research unlocks the hidden potential of metasurfaces August 13th, 2021
Metasurfaces control polarized light at will: New research unlocks the hidden potential of metasurfaces August 13th, 2021
![]() Arrowhead Pharmaceuticals Announces Closing of Agreement with Takeda November 27th, 2020
Arrowhead Pharmaceuticals Announces Closing of Agreement with Takeda November 27th, 2020
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








