Home > Press > UCLA chemists report new nano flash welding
Abstract:
UCLA chemists report the discovery of a remarkable new nanoscale phenomenon: An ordinary camera flash causes the instantaneous welding together of nanofibers made of polyaniline.
UCLA chemists report new nano flash welding
Los Angeles, CA – October 28, 2004
UCLA chemists report the discovery of a remarkable new nanoscale phenomenon: An ordinary camera flash causes the instantaneous welding together of nanofibers made of polyaniline, a unique synthetic polymer that can be made in either a conducting or an insulating form. The discovery, which the chemists call "flash welding," is published in the November issue of the journal Nature Materials.
Numerous applications potentially could result from this research in such areas as chemical sensors, separation membranes and nano devices.
"We used an ordinary 35-millimeter camera, but you could also use a laser, or any other high-intensity light source," said Richard B. Kaner, UCLA professor of inorganic chemistry and materials science and engineering, and a member of the California NanoSystems Institute at UCLA.
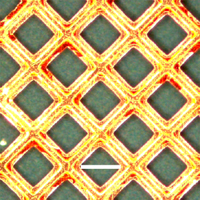
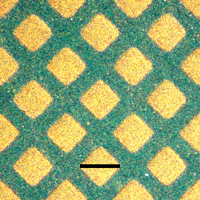
Click to enlarge. Optical microscope images show that flash welding through a copper grid reproduces the grid pattern on a polyaniline nanofiber film. The chemists put a copper grid on top of a nanofiber film (Figure 4a) and used it as a photomask for flash welding. After welding, they removed the mask and observed the grid pattern produced on the nanofiber film (Figure 4b). The areas exposed to the camera flash (the squares) are smooth and therefore appear bright gold because of the reflectance of incident light. The protected areas (the lines) remain as green nanofibers and appear darker because the rough surface scatters light and yields low reflectance. The high contrast between the resulting patterns is due to a change in reflectivity induced by flash welding. Courtesy and © Copyright UCLA |
"I was very surprised," Kaner said. "My graduate student, Jiaxing Huang, decided to take some pictures of his polyaniline nanofibers one evening when he heard a distinct popping sound and smelled burning plastic. Jiaxing recalled a paper that we had discussed during a group meeting reporting that carbon nanotubes burned up in response to a camera flash. By adjusting the distance of the camera flash to his material, he was able to produce smooth films with no burning, making this new discovery potentially useful."
The camera flash induces a chemical reaction; it starts a chain reaction in which the tiny nanofibers interact and cross-link, producing heat, which leads to more spontaneous cross-linking across the entire surface of the nanofibers, welding them together, Kaner said. Unlike carbon nanotubes, which burn up, this material is thermally absorbent and can dissipate the heat well enough so that it does not burn.
"We can envision welding other materials together as well," Kaner added. "One way to do this is to take two blocks of a conventional polymer and insert polyaniline nanofibers between them, then induce the cross-linking reaction to produce enough heat to weld the polymer blocks together. We can weld polyaniline to itself or to another polymer or potentially use it to join conventional polymers together." (A polymer is a long chain of molecules, commonly known as plastics.)
Because only the part exposed to light welds together, chemists can create patterns by covering sections that they do not want welded; they can control what parts weld together.
Kaner's research team searched for whether any conventional techniques have this same welding property. They found a recent commercial process called laser welding, now used in the electronics industry, in which a laser beam is used to weld together conventional polymers. "The trouble with laser welding," Kaner said, "is that lasers generally have a small cross-section and consume a lot of power. Our research has the potential of revolutionizing this process."
Nanofibers have high surface areas and important properties, from sensing to flash welding. "This shows why nano is important," Kaner said. "Here's a good example of where nano materials possess a property that conventional materials do not have."
Kaner and Huang were the first chemists to produce large quantities of pure polyaniline nanofibers, which can also be used for sensors -- findings they published last year in collaboration with Dr. Bruce Weiller and Shabnam Virji at Aerospace Corp. The nanofibers have a much greater response in a shorter time than sensors made with conventional polyaniline.
Jiaxing Huang has started a UC Berkeley postdoctoral fellowship.
The research is funded by the Microelectronics Advanced Research Corp.
Stuart Wolpert
stuartw@college.ucla.edu
310-206-0511
University of California - Los Angeles
Copyright © UCLA
If you have a comment, please us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Microelectronics Advanced Research Corp (MARCO)
Microelectronics Advanced Research Corp (MARCO)
| Related News Press |
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||