Home > Introduction > Nanotubes and Buckyballs
Nanotubes and Buckyballs
Last Updated: Monday, 20-Apr-2015 19:51:34 PDTGo directly to Websites
Nanotube:
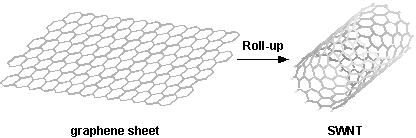
"Conceptually, single-wall carbon nanotubes (SWCNTs) can be considered to be formed by the rolling of a single layer of graphite (called a graphene layer) into a seamless cylinder. A multiwall carbon nanotube (MWCNT) can similarly be considered to be a coaxial assembly of cylinders of SWCNTs, like a Russian doll, one within another; the separation between tubes is about equal to that between the layers in natural graphite. Hence, nanotubes are one-dimensional objects with a well-defined direction along the nanotube axis that is analogous to the in-plane directions of graphite."
—M. S. Dresselhaus, Department of Physics and the Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology
A one dimensional fullerene (a convex cage of atoms with only hexagonal and/or pentagonal faces) with a cylindrical shape. Carbon nanotubes discovered in 1991 by Sumio Iijima resemble rolled up graphite, although they can not really be made that way. Depending on the direction that the tubes appear to have been rolled (quantified by the 'chiral vector'), they are known to act as conductors or semiconductors. Nanotubes are a proving to be useful as molecular components for nanotechnology. [Encyclopedia Nanotech]
Strictly speaking, any tube with nanoscale dimensions, but generally used to refer to carbon nanotubes, which are sheets of graphite rolled up to make a tube. A commonly mentioned non-carbon variety is made of boron nitride, another is silicon. These noncarbon nanotubes are most often referred to as nanowires. The dimensions are variable (down to 0.4 nm in diameter) and you can also get nanotubes within nanotubes, leading to a distinction between multi-walled and single-walled nanotubes. Apart from remarkable tensile strength, nanotubes exhibit varying electrical properties (depending on the way the graphite structure spirals around the tube, and other factors, such as doping), and can be superconducting, insulating, semiconducting or conducting (metallic). [CMP]
Nanotubes can be either electrically conductive or semiconductive, depending on their helicity, leading to nanoscale wires and electrical components. These one-dimensional fibers exhibit electrical conductivity as high as copper, thermal conductivity as high as diamond, strength 100 times greater than steel at one sixth the weight, and high strain to failure. NASA JSC - Carbon Nanotubes
A nanotube's chiral angle--the angle between the axis of its hexagonal pattern and the axis of the tube--determines whether the tube is metallic or semiconducting. Nanotubes Under Stress
A graphene sheet can be rolled more than one way, producing different types of carbon nanotubes. The three main types are armchair, zig-zag, and chiral. Examples
Copyright Professor Charles M. Lieber Group
And an excellent description of Carbon Nanotube Tips for Atomic Force Microscopy
Carbon nanotubes possess many unique properties which make them ideal AFM probes. Their high aspect ratio provides faithful imaging of deep trenches, while good resolution is retained due to their nanometer-scale diameter. These geometrical factors also lead to reduced tip-sample adhesion, which allows gentler imaging. Nanotubes elastically buckle rather than break when deformed, which results in highly robust probes. They are electrically conductive, which allows their use in STM and EFM (electric force microscopy), and they can be modified at their ends with specific chemical or biological groups for high resolution functional imaging. Professor Charles M. Lieber Group
CNT exhibits extraordinary mechanical properties: the Young's modulus is over 1 Tera Pascal. It is stiff as diamond. The estimated tensile strength is 200 Giga Pascal. These properties are ideal for reinforced composites, nanoelectromechanical systems (NEMS). Center for Nanotechnology | Gallery
Carbon Nanotube Transistors exploit the fact that nm- scale nanotubes (NT) are ready-made molecular wires and can be rendered into a conducting, semiconducting, or insulating state, which make them valuable for future nanocomputer design. ... Carbon nanotubes are quite popular now for their prospective electrical, thermal, and even selective-chemistry applications. Physics News 590, May 21, 2002
Many potential applications have been proposed for carbon nanotubes, including conductive and high-strength composites; energy storage and energy conversion devices; sensors; field emission displays and radiation sources; hydrogen storage media; and nanometer-sized semiconductor devices, probes, and interconnects. Some of these applications are now realized in products. Others are demonstrated in early to advanced devices, and one, hydrogen storage, is clouded by controversy. Nanotube cost, polydispersity in nanotube type, and limitations in processing and assembly methods are important barriers for some applications of single-walled nanotubes. Carbon Nanotubes—the Route Toward Applications Ray H. Baughman, Anvar A. Zakhidov, Walt A. de Heer
AKA: Multi-wall Carbon Nanotubes (MWNTs), Single-wall Carbon Nanotubes (SWCNs), (5, 5) armchair nanotube, (9, 0) zigzag nanotube, and (10, 5) chiral nanotube. Also, single-wall carbon nanotube field-effect transistors (CNFETs). See Nanotubes, Nanocones, and Nanosheets: an applet that lets you control in 3D the components and form elements. [Steffen Weber, PhD. See his VRML gallery of Fullerenes]. Also carbon nanowalls.

carbon nanotube with metal-semiconductor junction |
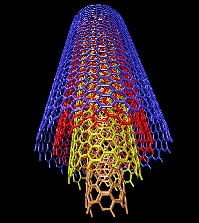
structure of a multi-walled nanotube |
Copyright Alain Rochefort Assistant Professor Engineering Physics Department,
Nanostructure Group, Center for Research on Computation and its Applications (CERCA).
Bucky Ball:
"It is the roundest and most symmetrical large molecule known to man. Buckministerfullerine continues to astonish with one amazing property after another. Named after American architect R. Buckminister Fuller who designed a geodesic dome with the same fundamental symmetry, C60 is the third major form of pure carbon; graphite and diamond are the other two." Bucky Balls - Andy Gion.
AKA: C60 molecules & buckminsterfullerene. Molecules made up of 60 carbon atoms arranged in a series of interlocking hexagons and pentagons, forming a structure that looks similar to a soccer ball
[Steffen Weber, PhD.]. C60 is actually a "truncated icosahedron", consisting of 12 pentagons and 20 hexagons. It was discovered in 1985 by Professor Sir Harry Kroto, and two Rice University professors, chemists Dr. Richard E. Smalley and Dr. Robert F. Curl Jr., [for which they were jointly awarded the 1996 Nobel Lauriate for chemistry] and is the only molecule composed of a single element to form a hollow spheroid [which gives the potential for filling it, and using it for novel drug-delivery systems. See
Structure of a New Family of Buckyballs Created].
"The buckyball, being the roundest of round molecules, is also quite resistant to high speed collisions. In fact, the buckyball can withstand slamming into a stainless steel plate at 15,000 mph, merely bouncing back, unharmed. When compressed to 70 percent of its original size, the buckyball
becomes more than twice as hard as its cousin, diamond." The Buckyball
- Rodrigo de Almeida Siqueira.
AKA: Endohedral fullerenes, carbon cages.

Click to enlarge Copyright Oliver Kreylos, Center for Image Processing and Integrated Computing (CIPIC), University of California, Davis. |
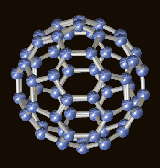
Click to enlarge Copyright Dr. Roger C. Wagner, Dept. of Biological Sciences, University of Delaware. |
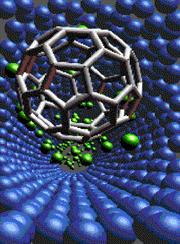
Click to enlarge Copyright ORNL. See Materials by Computational Design and Atomistic Simulations. This figure presents a visualization of a nanohydraulic piston. The model consists of a Carbon nanotube (blue), Helium atoms (green), and a "Buckyball" molecule. It is used to explore the stability of the system. |
Below you will find a selection of sites whose main theme is Nanotubes & carbon buckyballs. If you have another favorite, please us.
Back To Top
Nanotube, nanowhiskers, nanofibres, and buckyball links:
"NASA and the Johnson Space Center (JSC) have made a commitment to pursue and drive breakthrough technologies to expand human exploration of space. The very future of space exploration depends on advanced technologies such as nanotechnology and biomimetics. Toward this goal, JSC is focusing on the development of nanotechnology based on single-wall carbon nanotubes."
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||