Home > Nanotechnology Columns > UAlbany College of Nanoscale Science and Engineering > Super Acid Amplifiers for Extreme Ultraviolet (EUV) Lithography
 |
Robert Brainard CNSE Professor of Nanoscience |
Abstract:
For 40 years, the microelectronics industry has built integrated circuits with two-fold improvements in coprocessor speed every two years. This remarkable record of success is known as Moore's Law1 and has been driven by the industry's continued advances in packing more computing power (i.e. number of transistors) into each chip. Central to this success has been the use of a progression of shorter and shorter wavelengths of light during fabrication to provide a steady increase in the ability to print smaller and smaller features in these integrated circuits.
July 5th, 2011
Super Acid Amplifiers for Extreme Ultraviolet (EUV) Lithography
Moore's Law and EUV Lithography. For 40 years, the microelectronics industry has built integrated circuits with two-fold improvements in coprocessor speed every two years. This remarkable record of success is known as Moore's Law1 and has been driven by the industry's continued advances in packing more computing power (i.e. number of transistors) into each chip. Central to this success has been the use of a progression of shorter and shorter wavelengths of light during fabrication to provide a steady increase in the ability to print smaller and smaller features in these integrated circuits. Today's fastest computers are being fabricated using 193 nm light. The next wavelength of light to be used for high volume manufacture of the most advanced integrated circuits will be 13.5 nm light (called extreme ultraviolet (EUV) light). The College of Nanoscale Science and Engineering is one of the most important facilities in the world for EUV lithography research as it has a total of four EUV exposure tools capable of performing critical experiments with EUV photoresists.
Chemically Amplified Photoresists for EUV. High resolution chemically amplified photoresists2 are central to the manufacture of today's integrated circuits. These materials employ strong acids generated during exposure to 248, 193, or 13.5 nm light, which catalyze the transformation of photoresist polymers from insoluble to soluble in alkaline developers. The chemically amplified photoresists being invented today for use with extreme ultraviolet (EUV, 13.5 nm) light must simultaneously exhibit three properties: high resolution, low line edge roughness (LER), and high sensitivity.3-5
Acid Amplifiers. We have proposed that the best way to simultaneously improve these three properties in EUV resists is to increase the number of strong acids generated during exposure3 and we assert that acid amplifiers may be one of the best ways to achieve this goal. Acid amplifiers (AAs) are compounds that decompose via acid-catalyzed mechanisms to produce more acid (Figure 1).6 The idea, therefore, is to use AAs to catalytically amplify the signal produced by the EUV light during exposure.
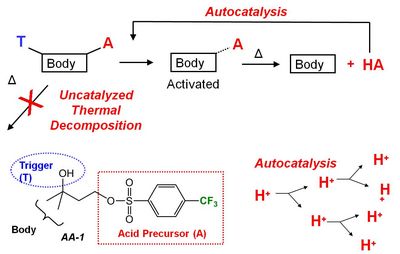 |
| Figure 1. Generic description of acid amplifiers developed by our group. |
Acid amplifiers have been known since 1995.7 Our contribution has been to specifically design AAs that generate super strong acids, since these super acids make the best catalysts for EUV resists. A primary challenge of making AAs that generate super acids is to make them stable, since stronger acids typically make weaker bonds to the rest of the AA molecule. Before we began our work, there were only two AAs that generate super acids previously reported in the literature. To date, we have designed and synthesized ~45 new AAs that generate super acids—dramatically increasing the number of acid amplifiers suitable for use with EUV photoresists.
To be successful in EUV photoresists, AAs must: (A) be thermally stable in the absence of catalytic acid; (B) rapidly decompose autocatalytically in the presence of the acids they produce; (C) give an overall improvement in the lithographic performance of the resist. Here, we report on the performance of five new compounds with respect to these three criteria (Figure 2). Four act as acid amplifiers and autocatalytically generate super acids while the fifth is too unstable to be used under normal processing conditions.8
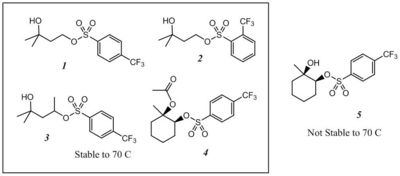 |
| Figure 2. Chemical Structures of four acid amplifiers (1-4) and one thermal acid generator (5). |
(A) Thermal Stability without Catalytic Acid. We use two methods to evaluate the thermal stability of AAs in the absence of acid. (1) We bake and develop resist films containing AAs using normal processing conditions. If an AA decomposes during the bake step, it creates a cascade of acid which in turn changes the solubility of the resist—if the AA is stable, the resist will not dissolve; if the AA decomposes, the resist is washed away by the developer.
We evaluated the thermal stability of the five compounds by baking resist films at 70 and 110 °C, followed by development.9 Table I shows that AAs 1 and 4 are stable to 110 °C, AAs 2 and 3 are stable to 70 °C, and 5 decomposes at 70 °C.
(2) We also prepare solutions containing an acid amplifier and a slight excess of base. We seal this solution into a special sample tube for use in a Nuclear Magnetic Resonance (NMR) instrument (same basic principle as Magnetic Resonance Imaging used in medical diagnostics). This NMR allows us to follow the kinetics of the reaction because we can exactly measure the concentration of the starting AA and the product acid, as a function of reaction time.10 The excess of base ensures that there is no build up of catalytic acid, and therefore we can get an accurate measurement of the rate of decomposition in the absence of acid. Figure 3 shows the concentration of AA-1 (actually ln[AA-1]) as a function of time at 100 °C.
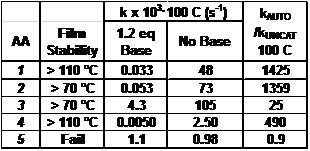 |
| Table I. Film stability and NMR kinetics results. |
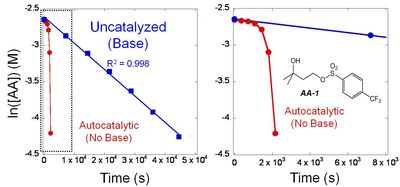 |
| Figure 3. Kinetic plots for the thermal decomposition of AA-1 in the presence and absence of base at 100 °C. |
(B) Acid Catalyzed Decomposition Rate. To determine the rate at which AA's decompose in the presence of catalytic acid, we prepare solutions of the AAs without base, in sealed NMR tubes. Using the NMR, we can follow the acid catalyzed decomposition rates. Figure 3 shows the rate at which AA-1 decomposes without base. The initial rate is the same as the uncatalyzed rate. Once the product acid is built up, the reaction occurs faster and faster. The two plots (with and without base) clearly show that the build-up of acid greatly accelerates the reaction rate. We extract the rate constants from these plots and characterize the AAs by the ratio of rate constants between the autocatalytic reaction (kAUTO) divided by the uncatalyzed thermal decomposition (kUNCAT). Since an important characteristic of AAs is that they react quickly in the presence of catalytic acid, and are stable in the absence of acid, high values of this ratio are essential. Table I shows that AAs 1, 2 and 4 have rate constants of ~500-1400. Two of the less-stable AAs (3 & 5) have rate ratios of 25 and 0.9. Clearly, AAs 1, 2 and 4 are the best choices for use in EUV lithography.
(C) Lithographic Performance. Ultimately, what matters most about acid amplifiers is their capacity for improving the resolution, LER and sensitivity of EUV resists. Figure 4 compares the lithographic performance of a resist with and without AA-4. The addition of AA clearly results in the simultaneous improvement in the resolution, LER and sensitivity of this resist.
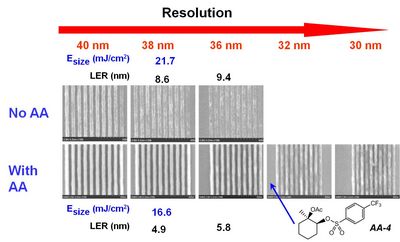 |
| Figure 4. Scanning electron micrographs of dense line images printed using a control resist formulation with and without 70 mM AA-4. |
Conclusions. We have compared the properties of five new compounds (1-5). Four of these are stable to at least 70 °C, and act as acid amplifiers with reactivity ratios of 50 to 1400. One of the compounds (AA-5) is not stable and decomposes thermally at 70 °C to make acid. We evaluated the catalyzed and uncatalyzed rate constants of these compounds and found that three of these AAs had rate ratios greater than 480. Lastly, we demonstrated that acid amplifiers are capable of simultaneously improving resolution, LER and sensitivity of EUV photoresists.
The development of acid amplifiers for use in EUV lithography continues to be an active area of research for our group. Current research is focused on improving the stability of AAs that generate strong super acids.
Acknowledgement. Many thanks to our coworkers: Seth Kruger, Sri Revuru, Craig Higgins, Sarah Gibbons, Daniel A. Freedman, Wang Yueh, Todd R. Younkin. This work was supported by Intel Corporation.
References.
1. Brenner, Alfred E.; Science 1997, 275(5306), 1551.
2. Ito, H.; Breyta, G.; Hofer, D.; Sooriyakumaran, R.; Petrillo, K.; Seeger, D. J. Photopoly. Sci. and Tech. 1994, 7, 433-47.
3. Brainard, R. L.; Trefonas, P.; Lammers, J. H.; Cutler, C. A.; Mackevich, J. F.; Trefonas, A.; Robertson, S. A. Proc. SPIE 2004, 5374(Pt. 1), 74-85.
4. Shumway, M. D.; Naulleau, P.; Goldberg, K. A.; Bokor, J. J. Vac. Sci. Tech. B 2005, 23, 2844-2847.
5. Gallatin, G. M.; Naulleau, P.; Niakoula, D.; Brainard, R. L.; Hassanein, E.; Matyi, R.; Thackeray, J.; Spear, K.; Dean, K. Proc. SPIE 2008, 6921, 69211E/1-69211E/11.
6. Ichimura, K. Chem. Record 2002, 2, 46-55.
7. K. Arimitsu, K. Kudo, H. Ohmori, K. Ichimura, "Sensitivity enhancement of chemical-amplification-type photoimaging materials by acetoacetic acid derivatives" J. Photopoly. Sci. Tech. 8(1), 43-4, (1995).1.
8. Kruger, S. A.; Revuru, S.; Higgins, C.; Gibbons, S.; Freedman, D. A.; Wang, Y.; Younkin, T.; Brainard, R. L. J. Am. Chem. Soc. 2009, 131(29), 9862-9863.
9. Photoresist films were prepared containing 70 mM of an acid amplifier, 7.5% of the photoacid generator bis(4-tert-butylphenyl)-iodonium nonaflate and phenolic terpolymer [poly(hydroxystyrene)-(styrene)(t-buylacrylate) 65/20/15 mol%] and 0.5 wt% of tetrabutyl ammonium hydroxide in 125 nm film on a silicon wafer. The film was then baked at 110 or 70 °C for 150 seconds and developed in 0.26 N Me4N+ OH- for 45 seconds.
10. Reaction rates (w/ and w/out 1.2 eq 2,4,6-tri-t-butylpyridine) were followed by integration of the CF3 peaks for the starting AA and the product CF3C6H4SO3H (or its pyridine salt). Estimated error for rate constants w/ and w/out base is 2.5% and 5%, respectively.
11. Second-order catalyzed reaction kinetics was evaluated by fitting data to Capellos, Christos; Benon Bielski. Kinetic Systems: Mathematical Description of Chemical Kinetics in Solution; Wiley-Interscience: New York, 1972.
Dr.Brainard's Bio
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








