Home > Nanotechnology Columns > UAlbany College of Nanoscale Science and Engineering > Neurotechnology - The advancement of Homo sapiens to Homo cyberneticus
 |
Matthew Hynd CNSE Assistant Professor of Nanobioscience University at Albany - College of Nanoscale Science & Engineering |
Abstract:
The convergence of neuroscience and nanotechnology holds promise for the successful development of electronic devices capable of directly interfacing with the central nervous system (CNS). In particular, neural prosthetic devices have become a powerful clinical strategy for the treatment of a variety of neurological disorders, including those sustained as a result of traumatic brain injury, epilepsy and Parkinson's disease (PD). Our research will have a substantive impact on the future abilities of health-care professionals to prevent or ameliorate the effects of neurological disorders using chronically-implanted neural prosthetic devices.
May 9th, 2010
Neurotechnology - The advancement of Homo sapiens to Homo cyberneticus
The human brain is often regarded as the most complex structure that is known to exist - be it manmade or biological. Encased within a skull that is only a quarter of an inch thick, the brain contains some 100 billion (1011) neurons and has over 100 trillion (1014) synapses for transmitting signals between neurons. The true nature of biological complexity can only be fully appreciated when you realize that these signals travel can travel over a combined 110,000 miles (176,000 km) of axons in the brain - in comparison this is half the average distance from the center of the Earth to the center of the Moon (238,857 miles).
My research group at the College of Nanoscale Science and Engineering (CNSE) is devoted to neurotechnology research and development. Neurotechnology can be broadly defined as any technology that has a fundamental influence on how people understand the brain and various aspects of consciousness, thought, and higher order activities in the brain. This emerging field includes the development of technologies that are designed to improve and repair brain function. Neurotechnology uses techniques and concepts from a variety of nanoscale disciplines including nanobiotechnology, nanomedicine, nanofabrication, nanomaterials and nanoeconomics.
Over the last decade considerable progress has been made in the fabrication of electronic devices at both the micro- and nano-scales for interfacing with the CNS (see figure 1). These neural prosthetic devices have become a powerful clinical strategy for the treatment of a variety of neurological disorders, including those sustained as a result of traumatic brain injury, epilepsy and Parkinson's disease (PD). In particular, deep brain stimulation (DBS) using neural prosthetic devices have provided remarkable therapeutic benefit to individuals suffering from otherwise treatment-resistant PD. To date, more than 35,000 patients worldwide have been implanted with neural prosthetic devices with over 250 centers in the U.S. performing the operation. However, while these devices have demonstrated immense potential for acute or short-term use in the brain, the ability to use these devices chronically has been largely unsuccessful. The insertion of neural prosthetic devices has been found to elicit reactive responses from both vasculature and nervous tissue. Our studies have shown that nervous tissue reacts to implanted silicon devices through a series of events, consisting of immediate-, early-phase-, and chronic-responses. The immediate-response (<1 hr) to devices begins within minutes following implantation. By 30 min post-insertion, neurons near the insertion site undergo morphological changes indicative of cellular degeneration. The early-phase response (1-7 days) is primarily inflammation, and includes damage to brain vasculature, activation of degenerative signaling cascades in neurons and glia and activation of resident microglia. Reduced cerebral blood flow following device insertion can also initiate a cascade of events leading to ischemia (insufficient blood flow to brain regions). The chronic response (>6 weeks) is characterized by a hyper-trophic reaction from reactive astrocytes. This results in the formation of a cellular sheath that functions as a diffusive barrier, insulating the device, and reducing the ability of the device to communicate with surrounding nervous tissue (see Figure 2). The response of the brain to the insertion of silicon devices can be compared to the biological responses following a localized injury outside of the CNS (e.g., getting a splinter in a finger). If the foreign body is not removed immediately, the body attempts to isolate it from causing further damage by surrounding it with numerous types of immune- and other reactive-cells. Ultimately similar responses prevent the successful, long-term integration of neural prosthetic devices into the brain.
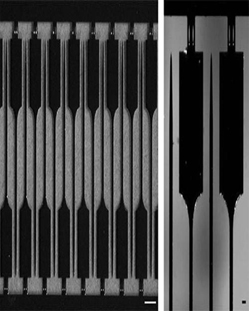 |
| Figure 1. Silicon-based neural prosthetic devices. Left panel scale bar = 500 μm. Right panel scale bar = 100 μm. |
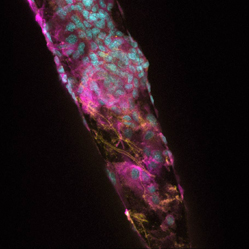 |
| Figure 2. Reactive cellular responses on a silicon neural prosthetic device following insertion in the brain. Labels: Yellow (glial cells), Magenta (microglial cells) and Cyan (cell nuclei). 60x magnification. Image by Jennifer King, Karen L. Smith, and Matthew R. Hynd |
My research group focuses on using nanoscale science to develop the next generation of tools and technologies to improve the clinical outcomes of neurological disorders using neural prosthetic devices. Our research into neurotechnology can be broadly classified into three main areas - i) device fabrication and development, ii) device biocompatibility, and iii) improving the integration of devices following their implantation into the brain. Some of our current projects include the development of optically transparent, polymer-based neural prosthetic devices and delivery of therapeutics and nucleic acids (e.g., short interfering RNA (siRNA) using microfluidic-based neural prosthetic devices (see figure 3). One of the most cutting-edge projects is funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB). This project is developing the world's first series of cell-based neural prosthetic devices. Rather than using passive electrodes to establish our electrical connection with the brain, we are using biologically-inspired design principles in these devices. These "hybrid" neural prosthetic devices contain neurons integrated with the devices, which following implantation, outgrow and directly interface with the host CNS. In this way we can use a biology-to-biology paradigm to enhance biocompatibility and long-term device performance. We are also developing a unique series of nanoscale devices in collaboration with Dr. Ji-Ung Lee, CNSE Empire Innovation Professor of Nanoscale Engineering. These devices are fabricated using vertically-aligned arrays of carbon nanotubes (CNT). Each CNT is 1-2 mm in length but composed of elements that are ~10 nm in diameter. These devices are 10,000x smaller than currently used silicon-based devices.
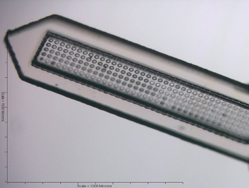 |
| Figure 3. The next generation of optically transparent, polymer-based neural prosthetic devices fabricated at CNSE. |
According to the World Health Organization (WHO) neurodegenerative disorders will become the world's second leading cause of death by the year 2040, overtaking deaths caused by both cancer and HIV/AIDS. The potential for biomedical applications and clinical remedies deriving from discoveries related to neurotechnology is therefore extremely high. In the year 2013, the market for neurological medical devices is estimated to exceed US$10 billion and is projected to grow at a rate of 20 to 30% in the next decade, far outpacing the market for cardiac devices. By 2040, collective sales of neural prosthetic devices will be over $30 billion annually. Presently, the USA is the world's single largest product market for neural prosthetic devices. Breakthroughs from our research at CNSE will therefore have a significant impact on the market for enabling new neural prosthetic-based technologies. By enhancing the long-term performance of neural prosthetic devices, our research will have enormous implications for society by facilitating a revolutionary change in the quality of life for people with CNS, sensory and/or motor deficits
Professor Hynd's Bio
Professor Hynd's Website
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








